How to Choose A Disinfectant: Make an Informed Decision

By Vince Battaglia
From the archives of The Canine Chronicle, October, 2017
Which statements are True or False?
• “Faster” means the product is stronger and kills more. T or F?
• Cleaning and disinfecting are the same thing. T or F?
• All disinfectants are created equal and can be used in the same way. T or F?
• If a disinfectant kills most germs, it must be toxic. T or F?
• More is better. T or F?
THE ANSWER TO ALL OF THE ABOVE IS FALSE.
While the statements above may sound logical and convincing, basing your disinfection program on these misconceptions can put your animals in danger. The disinfectants you use may not actually kill the pathogen that is infecting your space. So, instead, the most important question you can ask is “what is the affinities of the product you’re using to kill the specific pathogen threatening your kennel?”
We know that the lethal action of disinfectants for different type pathogens (bacteria, fungi, protozoa, viruses) depends on the chemical composition of the disinfectant and the make-up of the organism.
Therefore, when choosing a disinfectant, consider these characteristics:
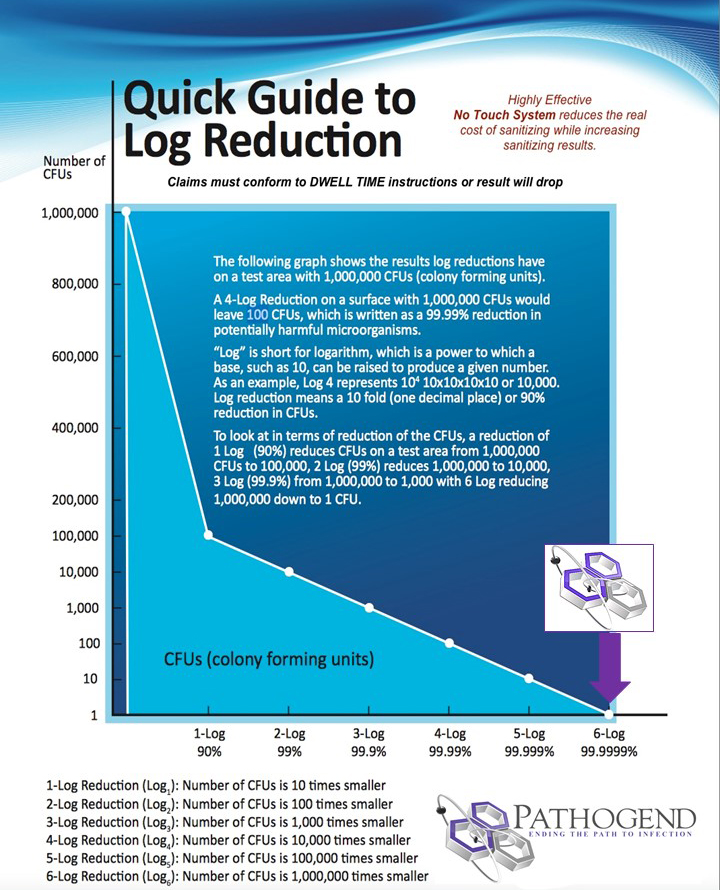
Efficacy. (killing efficiency against viruses, bacteria, fungi). Will it kill the target pathogen you want? The higher the log kill, the better. 99.9% = 3 log, 99.99% = 4 log, 99.999% = 5 log, 99.9999% = 6 log, followed by sterilization.
Contact time. Contact or dwell time is the most important. How long does a product need to stay wet to accomplish its end kill rate? Most dwell times are 10-30 minutes.
Cost. You get what you pay for.
Activity with organic matter. Will the product harm materials in your kennels? Some will over time. Bleach is known to be corrosive to metals and can cause damage to some plastics.
Residual activity. Is there anything left behind? Does one need to rinse treated areas off after using or will it brake down to natural materials that cannot be reactive?
Toxicity. How safe is it after uses? Is the residue harmful, can it be reactivated? Is it a respiratory irritant? Can it cause asthma? Is the product a Carcinogen?
Solubility (acidity, alkalinity, pH). What is the pH level of product? Not as important as the efficacy, but related.
Effect on fabric and metals. Read the label. Will the product, stain, weaken, or cause corrosive actions?
Temperature. Note the storage instructions. Are you keep your products safely away from pets and children? Note the window temperature range for best uses and best use by expiration date.
Disinfectants can be divided into the following classes based on their chemical composition:
Phenols
Quaternary ammonium
Hypochlorites (chlorine)
Oxidizing Agents (peroxide)
Potassium monopersulfate
Natural Disinfecting Agents
PHENOLS
Phenol (carbolic acid) is one of the oldest antiseptic agents. It is bacteriostatic at concentrations of 0.1%–1% and is bactericidal/fungicidal at 1%–2%. A 5% solution kills anthrax spores in 48 hours. The bactericidal activity is enhanced by EDTA and warm temperatures; it is decreased by alkaline medium (through ionization), lipids, soaps, and cold temperatures. Concentrations >0.5% exert a local anesthetic effect, whereas a 5% solution is strongly irritating and corrosive to tissues. CDC considers Phenol as Disinfectants some are intermediate and some are low level.
QUATERNARY AMMONIUM
Quaternary ammonium compounds are generally odorless, colorless, nonirritating, and deodorizing. They also have some detergent action, and they are good disinfectants. However, some quaternary ammonium compounds are inactivated in the presence of some soaps or soap residues, so careful product selection is important. Their antibacterial activity is reduced in the presence of organic material. Quaternary ammonium compounds are effective against bacteria and somewhat effective against fungi and viruses. CDC considers Ammoniums Low Level Disinfectants.
HYPOCHLORITES
Chlorine compounds are good disinfectants on clean surfaces, but are quickly inactivated by dirt. Chlorine is effective against bacteria and many viruses. These compounds are also much more active in warm water than in cold water. Chlorine solutions can be somewhat irritating to skin and corrosive to metal. They are relatively inexpensive. Surfaces must be pre-cleaned. Mixing with ammonia, ammonium quaternary compounds and other acidic products can create poisonous gas. May damage floor finishes, carpets, clothing and other fibers when used in higher concentrations. CDC considers Hypochlorites Intermediate Level Disinfectants.
OXIDIZING AGENTS
Accelerated hydrogen peroxide formulations are synergistic blends of 0.5%–2% hydrogen peroxide with anionic and nonionic surfactants and stabilizers that possess broad-spectrum antimicrobial activity. They are effective against bacteria, spores, mycobacteria, viruses, and fungi, with short contact times. Accelerated hydrogen peroxide formulations are nonirritating to eyes and skin and are biodegradable, decomposing to water and oxygen with no active chemical residues. CDC considers Oxidizing Agents to be Intermediate Level Disinfectant and Product Specific.
POTASSIUM MONOPERSULFATE
Potassium monopersulfate is the active ingredient in most non-chlorine shock products designed for use in swimming pools, and it’s the active ingredient in essentially all non-chlorine shock products formulated for use in spas and hot tubs. It is also referred to as potassium peroxymonosulfate or, simply, monopersulfate. Mainly used in shock pools and animal disinfections.
Natural Disinfecting Agents
The natural forces that reduce the pathogen load in the environment are important and can often be used to our advantage. These include sunlight, heat, cold, drying (desiccation) and agitation. The ultraviolet rays of sunlight are tremendously potent in killing microorganisms. This is very helpful outside of buildings and is used regularly to disinfect drinking water in smaller third world countries, but unfortunately the ultraviolet rays can’t pass through glass or roofs or dust. Drying from fresh air and wind will also kill pathogens, particularly when they are exposed in the process of cleaning.
When selecting a disinfectant, choose one that targets the pathogen problem you have, follow the instructions and< I cannot emphasize enough, use the full dwell time before drying or rinsing (an extra step). This writer feels hydrogen peroxide is under used as a disinfectant. Government agencies, cities, and the agriculture industry often use hydrogen peroxide to clean water and vegetables before going to market. Depending on the percentage of hydrogen peroxide, it would be easy to get a 99.9999% kill ratio. Not only that, hydrogen peroxide is an environmentally friendly choice, since it breaks down to water and oxygen, hydrogen peroxide does not discolor below a 9%, and cannot be reactive like other chemicals.
In conclusion, when kennel environments are cleaned and disinfected regularly, the potential for infection decreases dramatically. I hope this article helps you make informed choices in keeping your kennels safe and healthy.
Contact: Vince Battaglia Phone: 678.575.2889
Short URL: http://caninechronicle.com/?p=134590
Comments are closed













